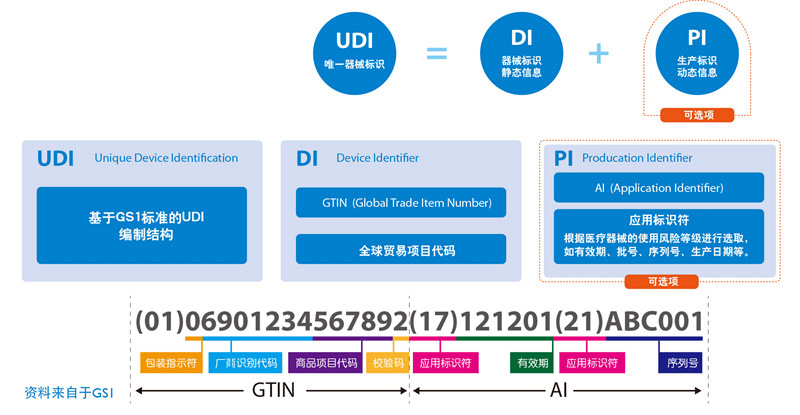
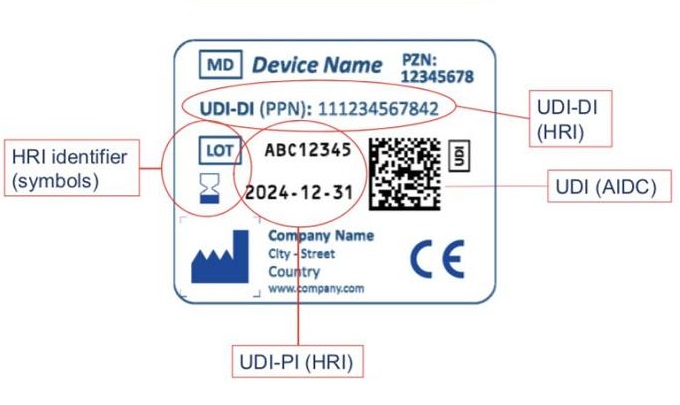
Rules of Digital Matrix Code UDI
The implementation process of UDI medical device regulations in countries and regions around the world
Unique Device Identification (UDI) is a "special medical device identification system" established by the US FDA. It is an identification given to a medical device throughout its life cycle, and is its unique "identity in the product supply chain." certificate". At present, advanced countries such as Europe and the United States have formulated UDI policies and regulations, stipulating or compulsory requirements that the UDI mark must be implemented on medical devices sold and circulated in their own countries.
· The U.S. Food and Drug Administration (FDA) officially promulgated the world-renowned medical device regulatory rules on September 23, 2013, that is, all medical devices sold in the United States must be marked with the UDI logo;
· In May 2017, the European Union promulgated the Medical Device Regulation MDR EU 2017/745, which stipulates that only medical device products with the unique device identification code UDI can enter the EU market legally and circulate freely;
· At the same time, China, Japan, Australia and other countries have also carried out related work one after another, and the global unique identification of medical devices is constantly advancing.